Quality Engineering Services
Program planning for operational excellence Qualification and validation consulting Validation protocol development and consulting Calibration program management Project management

Program planning for operational excellence Qualification and validation consulting Validation protocol development and consulting Calibration program management Project management
Coming Soon — Exclusive Customer Dashboard PK Calibration & Validation’s calibration software includes an exclusive customer dashboard. The dashboard enables: 24/7 access to view/manage assets Instant access to calibration certificates Audit readiness Total asset compliance and scheduling Fully validated and 21 CFR Part 11 compliance
Complete Service Offering PK Calibration & Validation’s capabilities include: Onsite calibration In-lab calibration Customized validation services to meet client needs Quality engineering Full range of calibration capabilities, including – Electronic calibrations (oscilloscopes, DMM’s, Hypots, High Voltage, Analyzers, and others) – Physical calibrations (Temperature, Humidity, Pressure, RPM, IR, Time, and others) – Dimensional calibrations (Calipers, Micrometers, […]
Accredited to Meet Strict Compliance Requirements PK Calibration & Validation holds the following accreditations: ISO/IEC 17025:2017 ANSI/NCSL Z540-1-1994 Certificate Number AC-1814 Accreditation Documentation: Palen Kimball ANAB Certificate of Accreditation ISO/IEC 17025:2017
Proven Instrument Calibration and Equipment Validation PK Calibration & Validation provides a full range of calibration. We specialize in servicing devices with third-party standards to meet, including ISO, FDA, cGMP, AMS 2750, NADCAP, CQI-9, and USDA. PK Calibration & Validation is accredited to the ISO/IEC 17025 standard through ANAB and is directly traceable to the […]

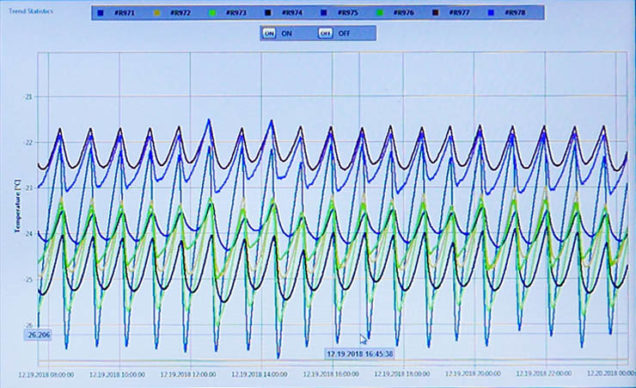
Temperature mapping validation State-of-the-art validation equipment Validation data points and parameters Capabilities from tabletop autoclaves to entire warehouses Full documentation and protocol services Reliable and repeatable results Full range of IQ/OQ/PQ validation support

17025 accredited Onsite N.I.S.T traceable calibrations Calibration program management – Various levels of service available ranging from on-demand to complete onsite program management – Environmental and process systems maintenance – Consulting services National field service program Comprehensive calibration and equipment validations Experienced metrologists 24/7 web support for audits and electronic certifications Onsite service minimizes clients’ […]

17025 accredited Calibration Lab N.I.S.T. traceability Clients can ship equipment to our lab from anywhere in the United States Full range of lab-based calibration services Over 24 years of experience in pharmaceutical, biotech, and medical device markets Knowledge of FDA audit requirements